Developmental Orthopedic Diseases (DOD) are the most common cause of lameness in growing dogs. Examples of DODs include:
- Hip and Elbow Dysplasia
- Osteochondrosis/ Osteochondritis Dissecans (OC/OCD)
- Avascular Necrosis of the Femoral Head
- Patellar Luxation
All of these conditions affect the joint(s) and will lead to osteoarthritis (OA).
Risk factors for developing DOD include
- Breed (genetics; large breed)
- Rapid growth (weight more than height)
- Nutritional factors such as free feeding/ overnutrition and improper vitamin and nutrient balance, including calcium and vitamin D.
- Early spay/neuter
Early diagnosis of joint disease offers the most effective means of minimizing the progression and symptoms of OA over a dog’s life. Since we know that DOD of the joints will lead to OA, identifying puppies with these conditions provides the optimal time to begin the discussion of OA management with the pet owner.
Below is a summary of each of the previously listed DOD. You will also find printable PDF versions of these topics that you can provide your client here (Developmental Orthopedic Disease Hip Dysplasia Form)
Hip Dysplasia
Hip dysplasia (HD) can be defined as abnormal development of the coxofemoral joint, resulting in hip laxity, or looseness, due to decreased coverage of the femoral head by the acetabulum and ineffective soft tissue stabilization of the joint. Over time, abnormal motion in the joint results in OA.
Hip dysplasia is a genetic condition but the actual development of clinical symptoms can be influenced by body weight/ body condition, reproductive status, age, conformation, and other environmental factors.
A study of genetically predisposed Labradors found that the development and progression of HD was significantly delayed or decreased in dogs that were maintained at a lean body condition through caloric restriction compared to litter-matched pairs of a higher body condition score. (Smith GK et al. JAVMA 2006)
Diagnosis
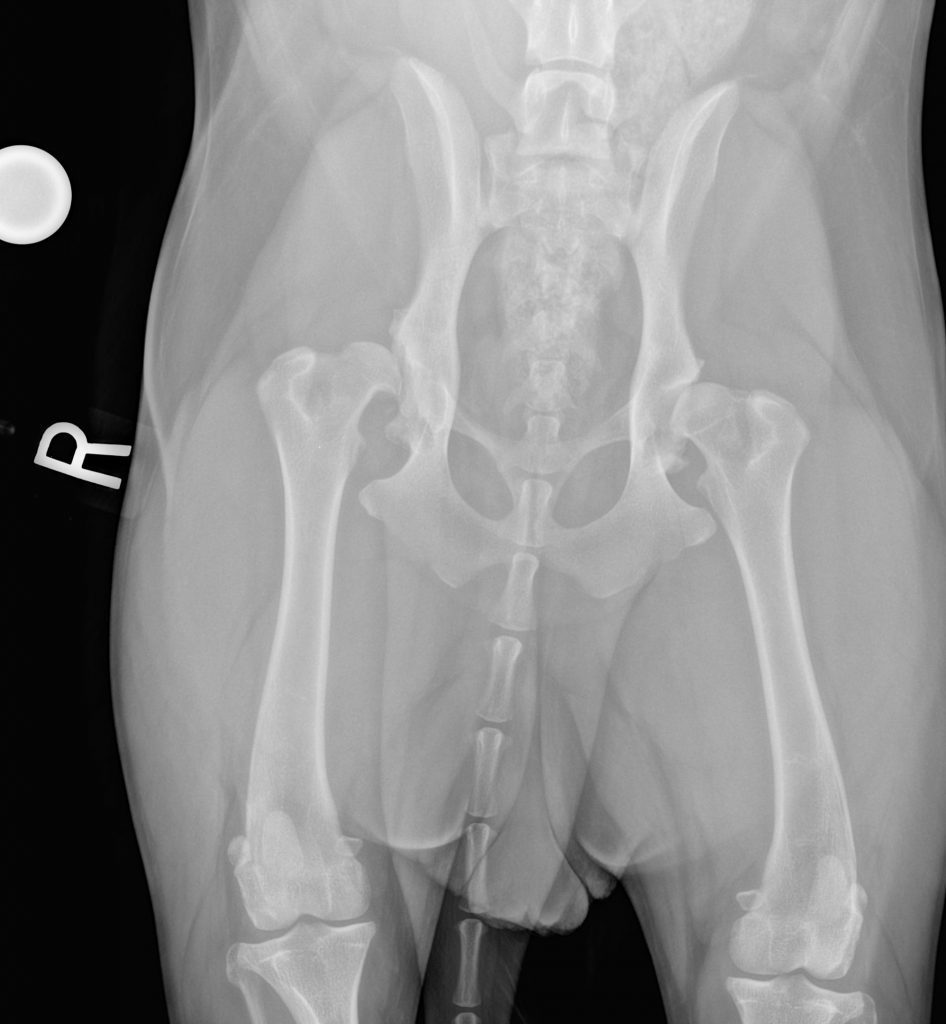
Dogs with HD often show clinical signs at two points in time: 4 months to 3-4 years and >7 years of age. Puppies and young dogs with HD present with a history of decreased activity or reluctance to play, jump or climb stairs, bunny hopping gait, hind limb muscle atrophy or underdevelopment, narrow hind limb stance, and pain or vocalization.
Palpation of the hips for an Ortolani sign can be performed in dogs as young as 16 weeks. A positive Ortolani means that the dog has hip laxity, ie, hip dysplasia.
PennHIP radiographs can be obtained at this age as well (Smith GK. Am J Vet Res, 1993). These radiographs objectively describe the degree of hip laxity and the likelihood of developing clinical signs of OA compared to other dogs in the breed. PennHIP is the most effective screening tool for identifying HD. (Powers MY. JAVMA 2010) The more common OFA hip view underestimates the degree of hip laxity and assigns a subjective score (excellent, good, fair). OFA films are not officially accepted until the dog is 2 years of age. 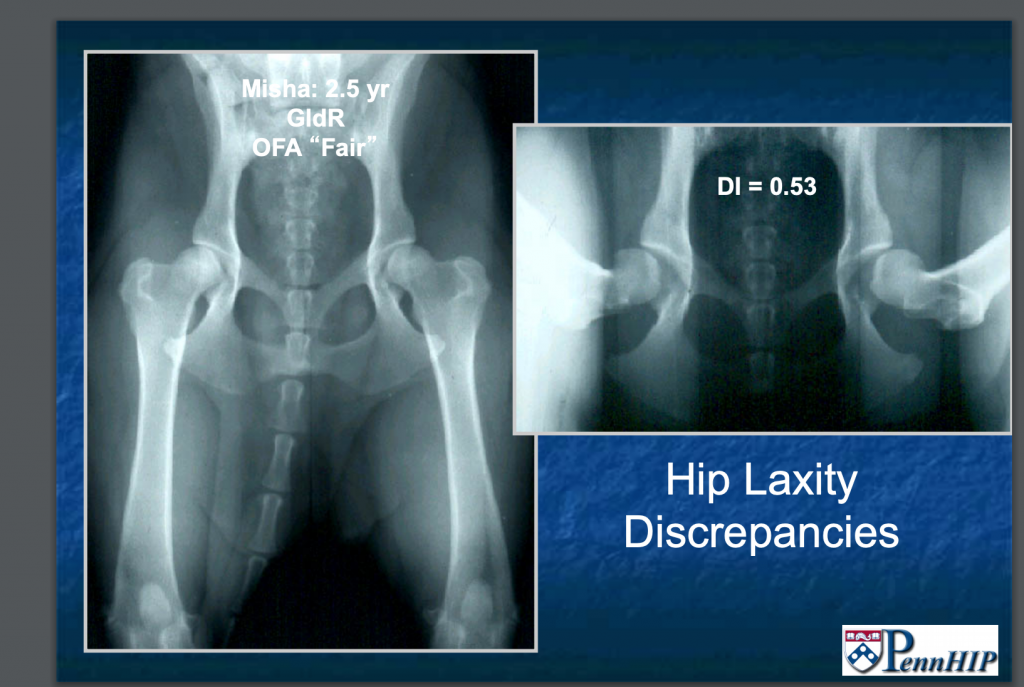
Mature dogs with symptoms related to arthritis of the hips show varying degrees of lameness that is worse after rest and heavy exercise, reluctance to jump, hind limb muscle atrophy, and behavior changes associated with pain.
The severity of HD and associated arthritis can be assessed radiographically; however, it is important to note that a direct correlation between radiographic severity and clinical signs does not exist. In other words, radiographs may show severe degenerative changes to the joint, yet the dog may be nearly asymptomatic (ie, “Dog’s don’t walk on their x-rays”). It is common for dogs with hip OA to develop compensatory changes in the lumbar spine and lumbosacral junction as the spine tries to compensate for the loss of range of motion in the hips.
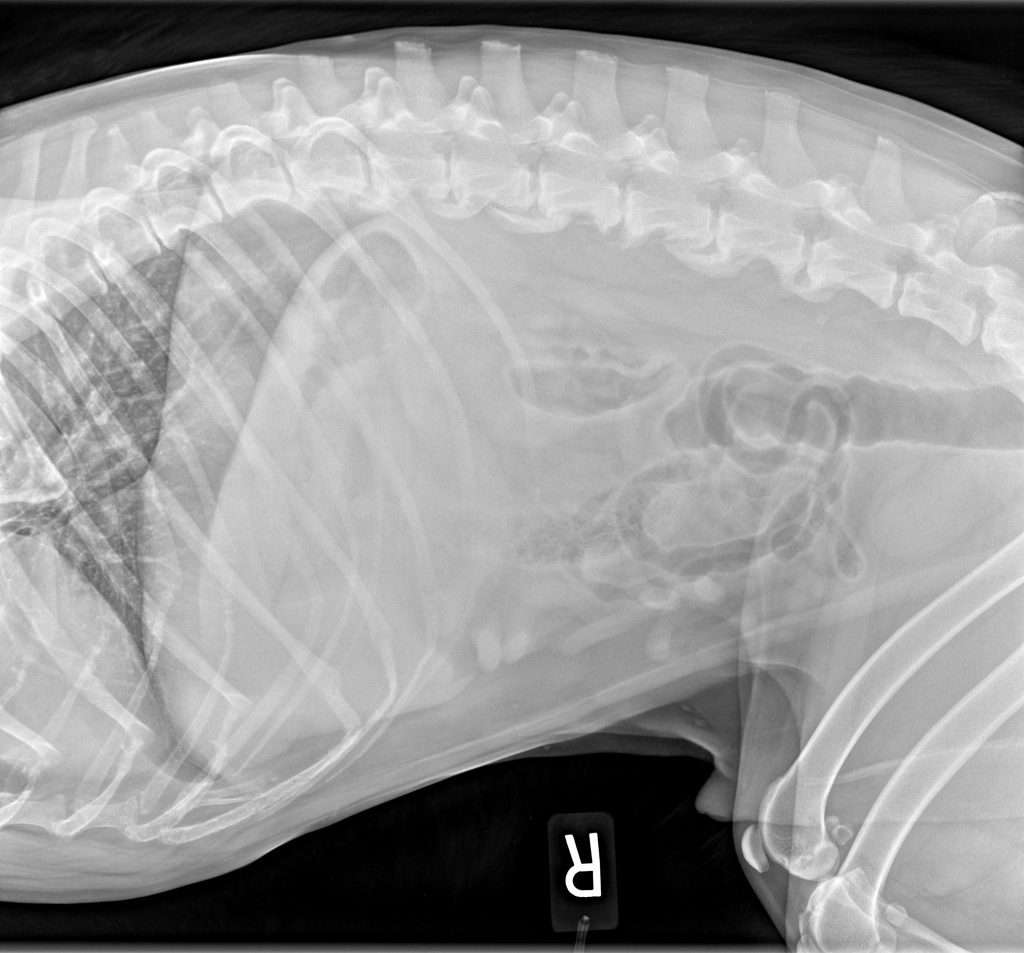
Treatment of HD
Non-surgical management
A diagnosis of HD/ OA does not imply the need for surgery. In fact, most dogs with HD and OA will be successfully managed with a multi-modal, non-surgical approach including activity modification, environmental adaptations, pain management, maintenance of a lean body condition, pharmacologic modulation of joint disease, therapeutic modalities, physical rehabilitation, intra-articular and complementary medicine. (Kirkby KA. Vet Surg 20120) Read more about a comprehensive treatment plan.
Surgical treatment
There are 4 types of surgical treatment for HD: two procedures are performed in juvenile dogs only with the goal of preventing the development of OA; two are typically performed in adult dogs that are symptomatic for OA.
- Juvenile Pubic Symphysiodesis (JPS):
- This is a relatively simple procedure that involves cauterizing the pubic symphysis in order to alter the growth of the acetabulum. When this is performed in puppies between 12-24 weeks of age, the development of the pelvis is improved so that the femoral head sits within the acetabulum and less arthritis can be expected long term. The JPS surgery is most effective for dogs with mild to moderate laxity and when performed before 20 weeks of age (but ideally between 12-16 weeks). Older puppies and those with severe laxity (distraction index, DI >0.70) may not benefit from the procedure. We do not recommend spaying or neutering client-owned dogs this young. However, should a dog have this procedure performed, it is advised that the client sign a consent that they will not breed their dog as the surgery does not prevent passing the genetic trait of HD on to offspring.
- Triple or Double Pelvic Osteotomy (TPO/DPO):
- This surgery involves cutting the pelvis in 2 or 3 places and rotating the acetabulum to provide better coverage of the femoral head. This is a more invasive procedure than the JPS. It is performed in puppies typically between 6-8 months of age. It can be effective in reducing the progression of arthritis but only if it performed prior to the onset of arthritis. Consultation with a surgeon is recommended to discuss this procedure further as this surgery has fallen out of favor for many surgeons.
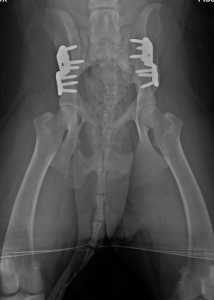
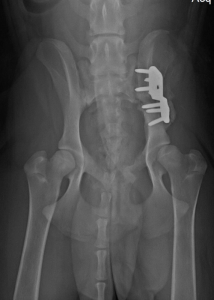
Treatment of arthritis secondary to HD is accomplished by either replacing the joint or removing the articulation and bone-on-bone contact. These surgeries are usually reserved for when non-surgical management efforts have been exhausted.
- Total Hip Replacement (THR):
- This is the gold standard surgery for HD, and results can be excellent with dogs returning to very active lives. However, there are potential risks associated with surgery. If non-surgical treatment of HD has been unsuccessful, consultation with a surgeon is recommended to discuss the pre-operative workup, post-operative care, and potential risks of THR.
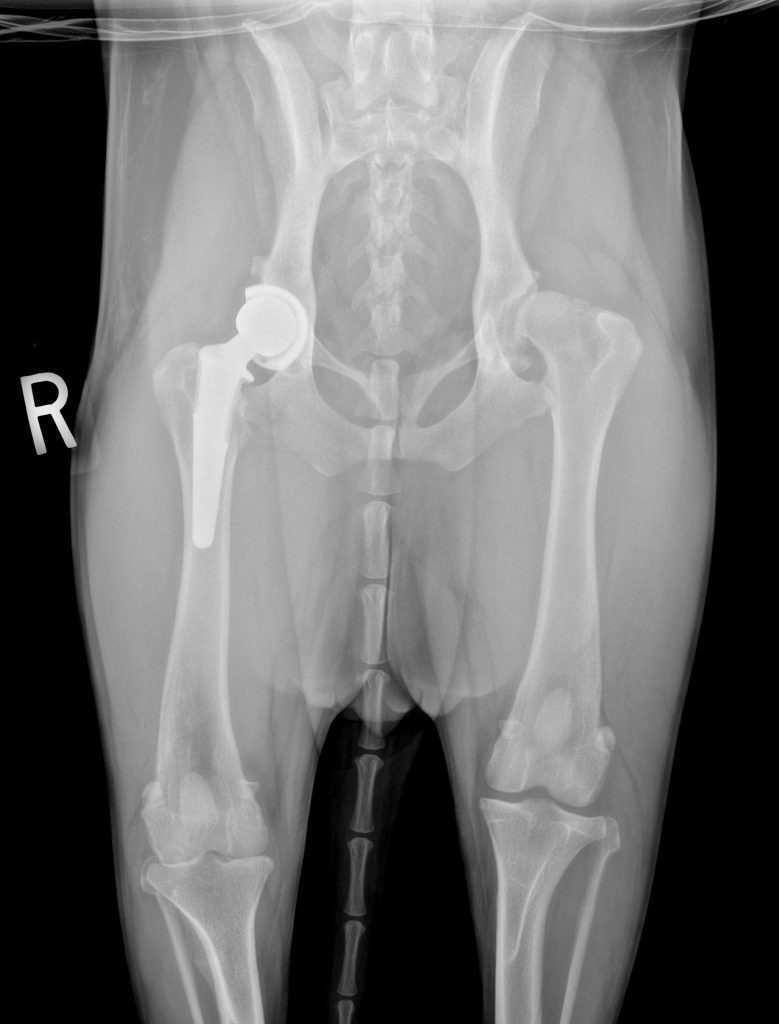
Total Hip Replacement
- This is the gold standard surgery for HD, and results can be excellent with dogs returning to very active lives. However, there are potential risks associated with surgery. If non-surgical treatment of HD has been unsuccessful, consultation with a surgeon is recommended to discuss the pre-operative workup, post-operative care, and potential risks of THR.
- Femoral Head and Neck Ostectomy (FHO): This procedure removes the femoral head and neck, thus removing the bone-on-bone contact of the hip. Many dogs do well with this procedure, but post-operative rehabilitation soon after surgery is crucial for optimal function, especially for large dogs.
Elbow Dysplasia
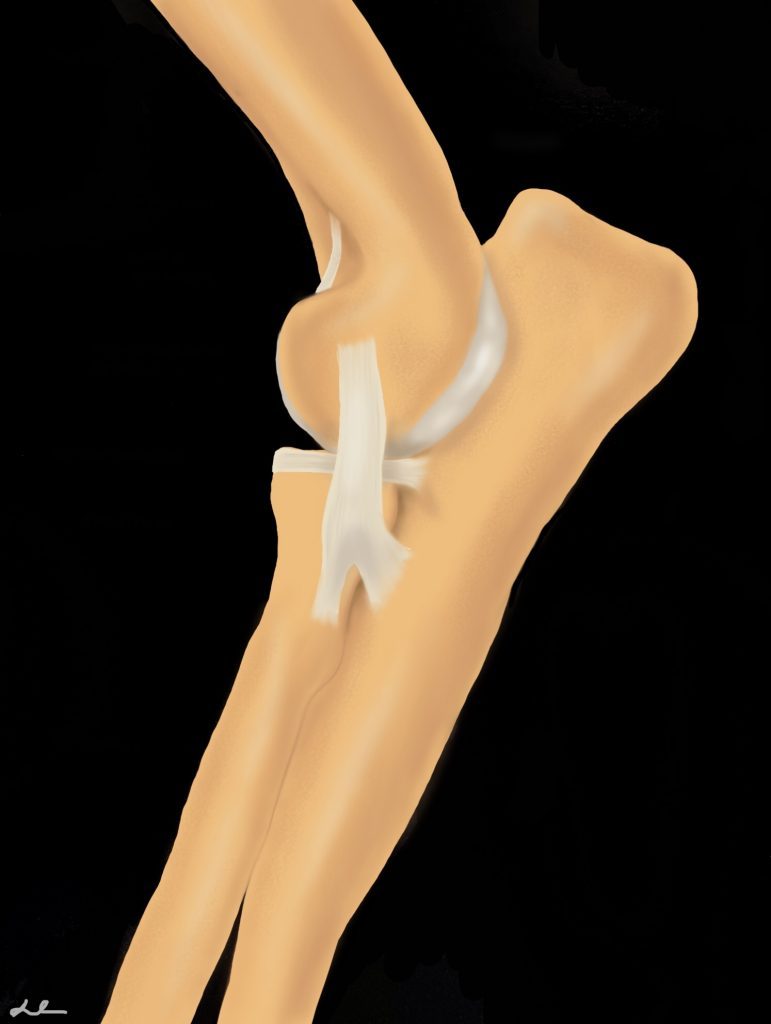
Elbow dysplasia is defined as abnormal development of the elbow joint. Three individual conditions are recognized as forms of ED:
- Fragmented Medial Coronoid Process (FMCP)
- Osteochondrosis/Osteochondritis dessicans (OC/OCD)
- Ununited Anconeal Process (UAP)
Dogs may have dysplasia in one elbow or both, and may have more than one type of pathology in the same elbow joint. All of these conditions will lead to OA in the joint.
Elbow dysplasia is considered a genetic condition but dietary factors and trauma may play a role in certain cases. Similar to HD, factors such as body weight/ condition and activity level can influence the development and progression of symptoms.
Treatment
Non-surgical
All forms of elbow dysplasia will result in OA, even with early surgical intervention. Surgical treatment of each condition is addressed below. However, long term, multimodal management of OA will be required and will include pain management, activity modification, environmental adaptations, weight management, rehabilitation techniques, and intra-articular therapies.
Surgical treatment of elbow OA
Unlike HD, joint replacement for elbow OA is not a highly successful procedure. There are surgeons continuing to work on refining the implants and surgical technique, but to date, total elbow replacement is not widely performed and carries a large number of risks.
Other surgical options for elbow arthritis in mature dogs include canine unicompartmental elbow replacement (CUE) and Sliding Humeral Osteotomy (SHO); these procedures are also not widely performed and have shown mixed results in clinical patients.
Consultation with your local surgeons is advised to learn their experience and recommendations for surgery in mature dogs with elbow OA.
Many surgeons feel that surgery is mostly indicated in young dogs with elbow dysplasia and non-surgical management may be recommended for older dogs with OA.
FRAGMENTED MEDIAL CORONOID PROCESS (FMCP)
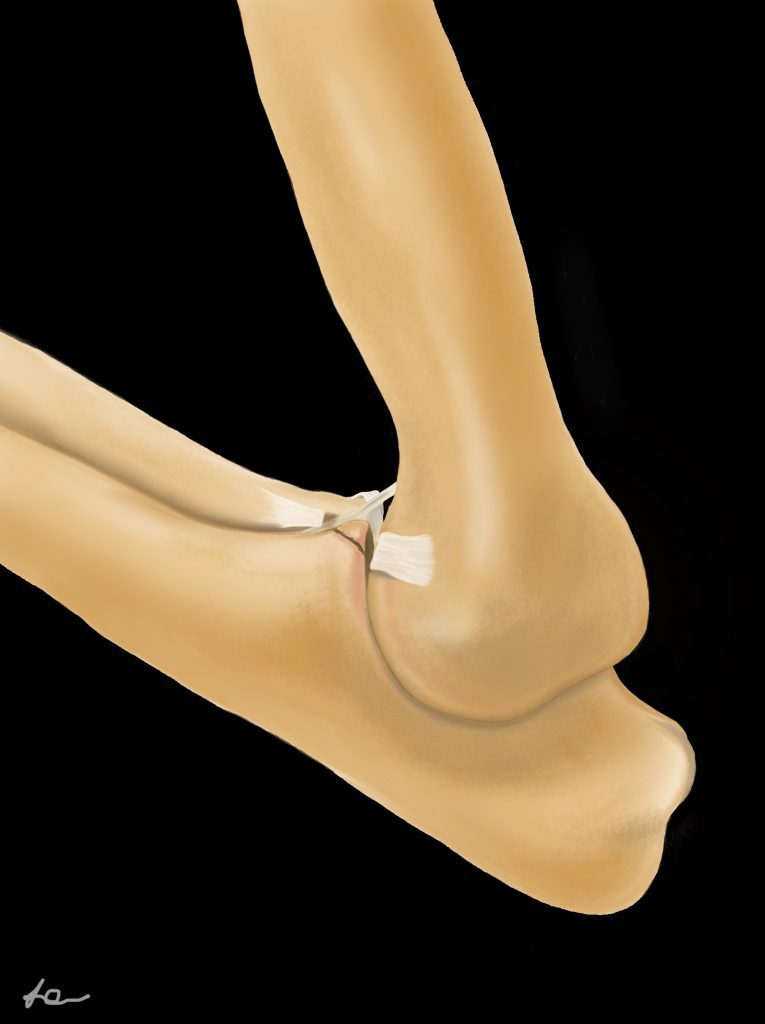
FMCP is the most common pathology present in cases of elbow dysplasia. It is characterized by fissures or complete separation of the medial coronoid process from the ulna. The exact pathophysiology is not fully understood, but it is believed that abnormal stresses placed on the coronoid process during development result in damage to the subchondral bone, and eventual fissuring or fracture. (Picture of FMCP) Stephanie image insert
Diagnosis
Clinical signs of FMCP begin between 5-8 months of age and include acute or chronic intermittent lameness, stiffness, and stilted gait. Lameness is usually intensified by exercise, and prominent after resting. In some cases, lameness is not apparent until later in life when significant OA has developed.
Effusion may be palpated in the joint and pain elicited upon full extension and flexion of the joint. Older dogs may have crepitus and significant loss of range of motion (typically loss of flexion more than extension). The clinical signs of FMCP are very similar to those of OCD, and it is likely that the two coexist in a third of cases. The majority of cases are bilateral, but clinical signs may be unilateral.
Radiographs may show degenerative changes in the elbow, including sclerosis of the ulna and osteophyte formation. It is difficult to visualize the coronoid process on radiographs, and FMCPs are rarely diagnosed on radiographs alone. Definitive diagnosis requires either CT or arthroscopy.
Surgical Treatment
There are several surgical options that may be considered for young dogs diagnosed with FMCP. These include: arthroscopic fragment removal, subtotal coronoidectomy, variations of ulnar osteotomy or ostectomy, and biceps ulnar release procedure (BURP). While most surgeons report improvement in lameness following fragment removal and other procedures, surgery will not be curative and long term management of arthritis will be required.
UNUNITED ANCONEAL PROCESS (UAP)
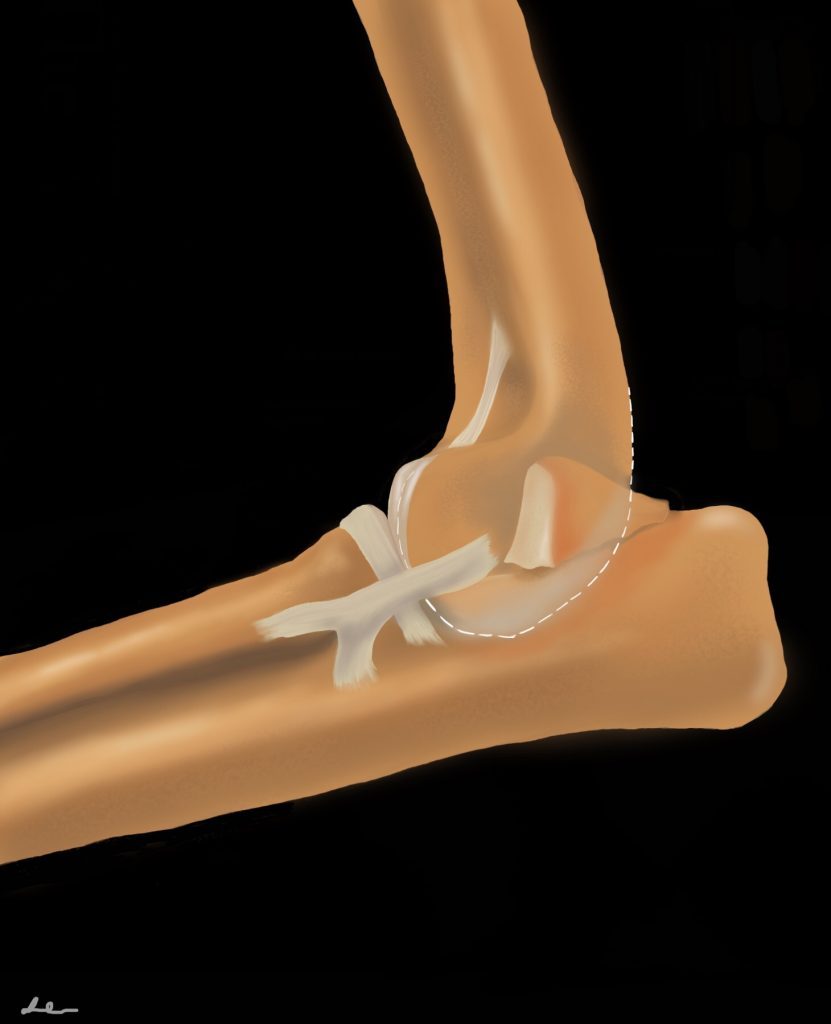
UAP is the failure of the physis of the anconeal process to fuse properly with the olecranon. The physis should fuse by 20 weeks of age; an open physis beyond this age indicates UAP (though confirmation at 24 weeks is often recommended). UAP leads to instability or detachment of the process and osteoarthritis of the elbow. Males are reportedly predisposed, and UAP occurs in both elbows approximately 25% of the time.
Diagnosis
UAP is most commonly diagnosed in German Shepherds, though it can develop in any breed, particularly large, giant, and chondrodystrophic breeds. Clinical signs are typically seen around 5-6 months age. Occasionally, lameness is not observed until the dog is several years old. In the earliest stages, the only clinical signs may be mild lameness and swelling on the lateral aspect of the elbow. Palpation of the joint in older dogs may reveal swelling and crepitus within the joint.
A preliminary diagnosis may be made by clinical signs, age and breed. The diagnosis will be confirmed by flexed lateral radiographs showing the partially or fully detached anconeal process, as well as possible osteophytes throughout the joint.
Treatment
There are several surgical options that may be considered for young dogs diagnosed with UAP. These include: arthroscopy to evaluate the entire joint, reattaching the anconeal process with a screw, ulnar osteotomy to relieve tension on the UAP so that it may fuse, or excision of the anconeal process. Like other components of elbow dysplasia, UAP will likely result in some degree of long term OA.
Osteochondrosis/ OSTEOCHONDRITIS DESSICANS (OCD)
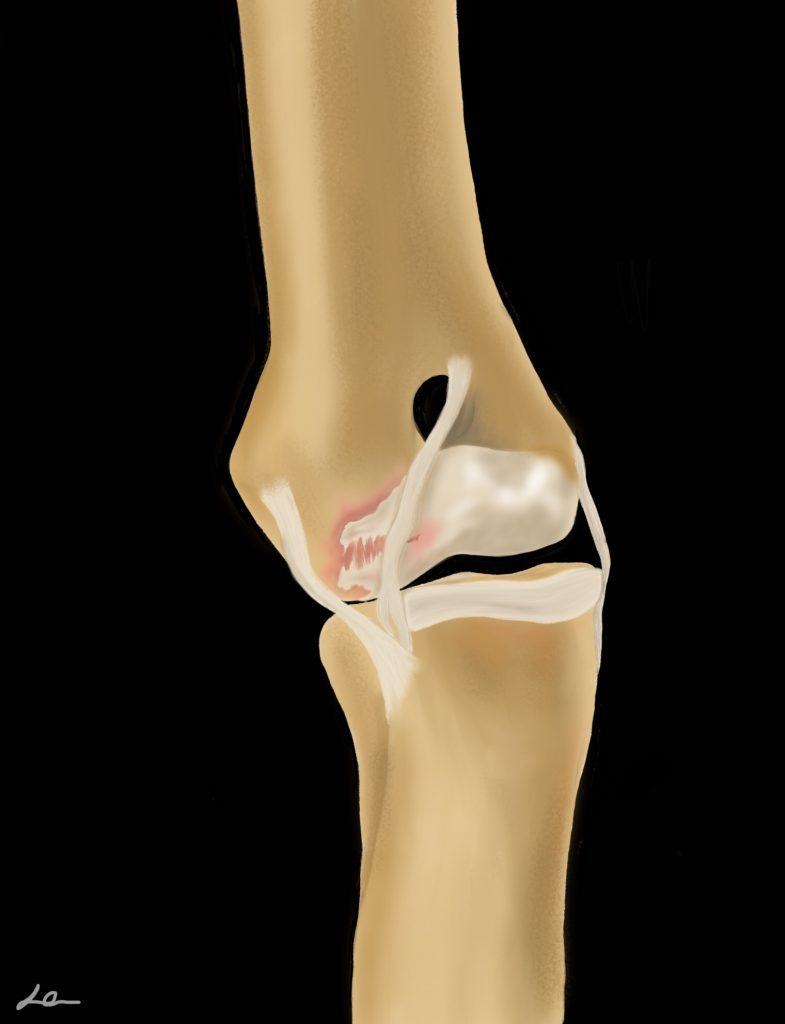
Osteochondrosis is a defect in endochondral ossification that results in a discrete area where cartilage fails to fully mature, is thickened, and weakly attached to underlying bone. Over time, the lesion may progress, forming a cartilage flap (osteochondritis dissecans, OCD). Once formed, this flap cannot heal back down to the underlying lesion bed. Instead, it will either continue to degenerate in place, or may break free and migrate to another area of the joint (“joint mice”). In either case, the presence of the flap will cause pain and inflammation within the joint. (Image of OC/OCD) Stephanie image
OC/OCD can develop in any joint, but the most common sites are shoulder (caudal humeral head), tarsus (medial trochlear ridge of the talus), stifle (lateral femoral condyle) and elbow (medial humeral condyle).
Diagnosis
No matter which joint OC/OCD affects, clinical signs typically begin between 5-8 months of age and include acute or chronic intermittent lameness, stiffness, and stilted gait. Lameness is usually intensified by exercise, and prominent after resting. In some cases, lameness is not apparent until later in life when significant OA has developed.
Effusion may be palpated in the stifle, tarsus and elbow joints; effusion will not be palpable in the shoulder. Tarsal OC/OCD typically results in a large amount of periarticular fibrosis that is easily identified (visually and palpably) on the medial aspect of the joint. Pain is often elicited upon full extension and flexion of the joints. Older dogs may have crepitus and significant loss of range of motion (typically loss of flexion more than extension).
Radiographs are typically sufficient to diagnose OC/OCD in the shoulder, stifle and tarsus, though advanced imaging may be needed in some cases. Radiographs may not show OC/OCD of the elbow and definitive diagnosis typically requires either CT or arthroscopy.
Treatment
Treatment for OCD consists of surgical (typically arthroscopic) debridement of the flap and abnormal cartilage. Then, the most common treatment is osteostixis, or the creation of small holes into the underlying subchondral bone to encourage in-growth of fibrocartilage (scar-tissue-type cartilage). Some surgeons will then inject PRP or other biologic therapies to encourage healing (though growth of normal hyaline cartilage has not been proven with these treatments).
Alternatively, a surgeon may elect to place an autologous, allogeneic or synthetic graft in the OCD defect. The autograph procedure is called Osteochondral Autograph Transfer System (OATS). Not all dogs with OC/OCD are candidates for these procedures, and long term results are mixed. Consultation with a local surgeon is advised to learn if this procedure is offered in your region.
It is important to realize that while surgery often provides improvement in lameness, this will not be a curative surgery and long term management of arthritis will be required.
Avascular Necrosis of the Femoral Head (Legg-Calve Perthes)
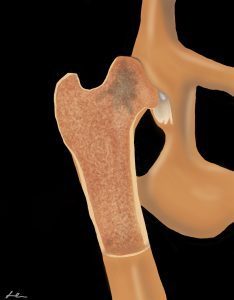
Avascular necrosis of the femoral head, also called Legg-Calve`-Perthes (LCP), is a DOD that typically occurs in toy breed dogs, such as Yorkshire Terriers. The cause of LCP is not completely known but may be due to trauma, infection, metabolic or hormonal imbalance, vascular abnormalities, or genetic predisposition. Disruption of the blood supply to the femoral head leads to the collapse of the subchondral bone underlying the cartilage of the femoral head, resulting in deformation of the femoral head and neck. The condition is typically unilateral, though it can occur bilaterally in approximately 15% of cases.
Diagnosis
LCP is most often seen in puppies between 4-11 months of age, with one study indicating the average age of presentation to be 7 months of age. Lameness may be acute or chronic and is often non-weight bearing. Most dogs have discomfort with manipulation of the hip joint. A diagnosis is usually easily confirmed on radiographs.
Treatment
The most common and successful treatment for LCP is femoral head and neck ostec
tomy (FHO). This surgery results in an excellent prognosis, with reports of relieving pain and lameness in 80-100% of dogs. Rehabilitation is strongly recommended following FHO.
Conservative/ non-surgical management is not recommended unless surgery is not feasible due to financial or medical limitations.
With successful FHO, hip OA is not expected to result in clinical symptoms. However, the foundational principles of OA management, including maintenance of a lean body condition and regular, controlled exercises, should be recommended.
Patella Luxation
Traumatic patella luxation can occur, but patella luxation is more often considered a congenital, developmental condition. Patella luxation results from abnormal development of the hip, femur and/or tibia and resultant abnormal biomechanics of the quadriceps extensor mechanism.
The quadriceps group originates at the proximal femur (rectus femoris originates on the cranial aspect of the acetabulum) and inserts onto the patella, which then inserts via the patella ligament onto the tibial tuberosity. The function of the patella is to improve the efficiency of the extensor mechanism. The quadriceps mechanism should form a straight line from the origin to insertion with the patella articulating with the trochlear groove of the femur. If abnormal growth occurs resulting in the femur bowing and/or the tibial tuberosity displaced medially or laterally, the patella will not be biomechanically stable within the trochlear groove. Quadriceps contraction will force the patella to subluxate or luxate over the trochlear ridged, the direction depending on the direction of the forces applied.
Distal femoral varus and/or medial tibial tuberosity displacement or internal tibial torsion leads to medial patella luxation. Distal femoral valgus and/or lateral tibial tuberosity displacement or external tibial torsion leads to lateral patella luxation. The tissues on the opposite side of luxation tend to stretch, while tissues on the side of luxation can contract. Over time, as the patella subluxates over the trochlear ridge, the ridge is worn down and the trochlear groove becomes more shallow. As such, patella luxation can progress in severity.
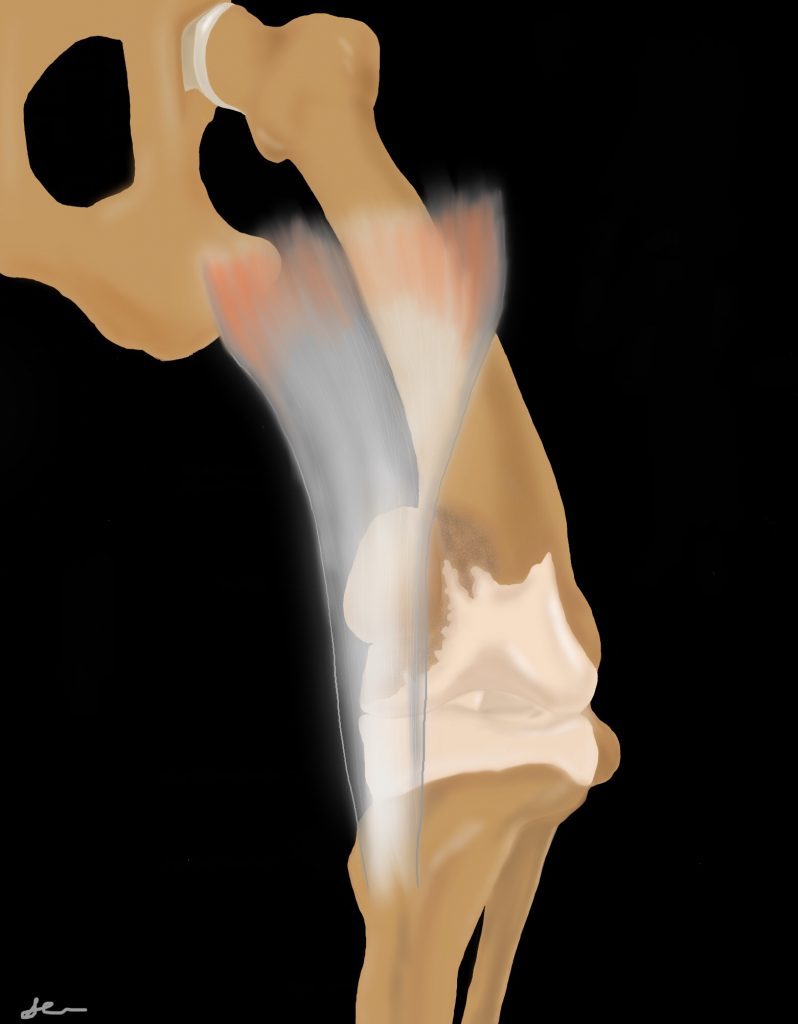
Medial patella luxation (MPL) is more common in all sizes of dogs than lateral patella luxation.
Certain breeds are predisposed to MPL, such as Yorkies, Pomeranians, Chihuahuas, and Pitbulls. Lateral patella luxation occurs more often in large breed dogs than small (though MPL is still more common than LPL in large breeds). The condition is frequently bilateral, though one side may be more severely affected than the other.
Diagnosis
Dogs with patella luxation typically show an intermittent, non-weight bearing lameness, characterized as a “skip” over several stride lengths. Usually, there is no lameness in between skips. MPL pre-disposes dogs to tearing of the cranial cruciate ligament (CCL). When this occurs, the degree of lameness typically worsens and the dog may be non-weight bearing.
The diagnosis of MPL or LPL is made on physical examination.
A grade is typically assigned:
I: Patella is located in the trochlear groove, with manual pressure and positioning of the limb, it can be completely luxated, but when pressure released, patella returns to trochlear groove
II: Patella is located in the trochlear groove, with manual pressure and positioning of the limb, it can be completely luxated. When pressure is released the patella remains luxated; with manipulation of the limb (flexion/ extension/ rotation), it returns to the trochlear groove.
III: The patella is palpably luxated, but can be manually repositioned in the trochlear groove.
IV: The patella is permanently luxated; cannot be replaced in the trochlear groove.
Radiographs are often obtained to evaluate the conformation of the bones and identify OA. If significant joint effusion is present, this suggests concurrent CCL disease.
Treatment
Surgical treatment is generally recommended for dogs with Grade III or IV MPL, frequent lameness associated with Grade II MPL, and animals with concurrent CCLR.
Surgical procedures aim to realign the quadriceps-patellar mechanism. While surgery is tailored to the individual patient, it usually involves trochleaplasty (wedge or block), re-alignment of the tibial tuberosity (tibial tuberosity transposition, anti-rotational suture), and imbrication of the stretched tissues (ie, lateral imbrication for MPL). Distal femoral osteotomy/ostectomy may be recommended for dogs with significant distal femoral varus.
Patella luxation has been shown to lead to stifle OA, particularly if CCL rupture occurs. Therefore, long term management of OA is recommended.
References
HIP
Evans HE. Arthrology. In: Miller’s Anatomy of the Dog 3rd ed. W.B. Saunders Co Philadelphia PA 1993
Dassler CL: Canine hip dysplasia: Diagnosis and nonsurgical treatment. In: Textbook of Small Animal Surgery 3rd edition. Saunders, Philadelphia, PA. pp 2019-2020, 2003.
Demko J, McLaughlin R. Developmental orthopedic disease. Vet Clin Small Anim 2005 35:1111-1135.
Witsberger TH, Villamil JA, Schultz LG, et al. Prevalence of and risk factors for hip dyplasia and cranial cruciate ligament deficiency in dogs J Am Vet Med Assoc 2008 232:1818–1824.
Smith GK, Lawler DF, Biery DN, et al. Chronology of hip dysplasia development in a cohort of 48 Labrador Retrievers followed for life. Vet Surg 2012.
Smith GK, Paster ER, Powers MY, et al. Lifelong diet restriction and radiographic evidence of osteoarthritis of the hip joint in dogs. JAVMA 2006
Krontveit RI, Trangerud C, Saevik BK, et al. Risk factors for hip-related clinical signs in a prospective cohort study of four large breeds in Norway. Prev Vet Med 2012.
Smith GK, Gregor TP, Rhodes WH, et al. Coxofemoral joint laxity from distraction radiography and its contemporaneous and prospective correlation with laxity, subjective score, and evidence of degenerative joint disease from conventional hip-extended radiography in dogs. Am J Vet Res 1993.
Powers MY, Karbe GT, Gregor TP, et al. Evaluation of the relationship between Orthopedic Foundation for Animals’ hip joint scores and PennHIP distraction index values in dogs. JAVMA 2010.
Barr ARS, Benny HR, Gibbs C: Clinical hip dysplasia in growing dogs: the long-term results of conservative management. J Small Anim Pract 28: 243-252, 1987.
Plante J, Dupuis J, Beauregard G, et al: Long-term results of conservative treatment, excision arthroplasty and triple pelvic osteotomy for the treatment of hip dysplasia in the immature dog. Vet Comp Orthop Trauma 10: 101-110, 1997.
Farrell M, Clements DN, Mellor D, et al: Retrospective evaluation of the long-term outcome of non-surgical management of 74 dogs with clinical hip dysplasia. Vet Rec 160: 506-511, 2007.
Felson DT, Chaisson CE: Understanding the relationship between body weight and osteoarthritis. Ballieres clin Rheumatol 11: 671-681, 1997.
Kirkby KA, Lewis DD. Canine hip dysplasia: Reviewing the evidence for non-surgical management. Vet Surg 2012
Dueland RT, Adams WM, Patricelli AJ, et al. Canine hip dysplasia treated by juvenile pubic symphysiodesis. Part 1: Two year results of computed tomography and distraction index. VCOT 2010.
Dueland RT, Adams WM, Patricelli AJ, et al. Canine hip dysplasia treated by juvenile pubic symphysiodesis. Part 2: Two year clinical results. VCOT 2010.
Vezzoni A, Dravelli G, Vezzoni L, et al. Comparison of conservative management and juvenile pubic symphysiodesis in the early treatment of canine hip dysplasia. VCOT 2008.
Slocum B, Devine T. Pelvic osteotomy for axial rotation of the acetabular segment in dogs with hip dysplasia. Vet Clin North Am Small Anim Pract 1992.
Johnson AL, Smith CW, Pijanowski GJ, Hungerford LL. Triple pelvic osteotomy: effect on limb function and progression of degenerative joint disease. J Am Anim
Hosp Assoc. 1998 May-Jun;34(3):260-4.
Vezzoni A, Boiocchi S, Vezzoni L, et al. Double pelvic osteotomy for the treatment of hip dysplasia in young dogs. VCOT 2010.
Conzemius MG, Vandervoort J. Total joint replacement in the dog. Vet Clin North Am Small Anim 2005
Piek CJ, Hazewinkel HA, Wolvekamp WT, et al. Long-term follow-up of avascular necrosis of the femoral head in the dog. J Small Anim Pract 1996 37(1):12-18.
Harper TAM. Femoral head and neck excision. Vet Clin North Am Small Anim Pract 2017.
ELBOW
Trostel CT, McLaughlin RM, Pool RR. Canine elbow dysplasia: anatomy and pathogenesis. Compend Contin Educ Pract Vet 2003 25 (10):754-761.
Schulz KS, Krotscheck U. Canine elbow dysplasia. In: Textbook of small animal surgery, 3rd ed. Ed. Slatter D. Philadelphia: W.B. Saunders, 2003 pp. 1927-1952.
Wind AP, Packard ME. Elbow incongruity and developmental elbow diseases in the dog: Part II. J Americ Anim Hosp Assoc 1986 22:725-730.
deBakker E, Samoy Y, Gielen I, VanRyssen B. Medial humeral epicondylar lesions in the canine elbow: A review of the literature. V Comp Orthop Traumatol 2011 24:9-17.
Corley EA, Sutherland TM, Carlsson WD. Genetic aspects of canine elbow dysplasia. J Am Vet Med Assoc 1968 153:543-547.
Krotscheck U, Hulse DA, Bahr A, Jerram RM. Ununited anconeal process: lag-screw fixation with proximal ulnar osteotomy. V Comp Ortho Trauma 13:212-216.
Meyer-Lindenberg A, Fehr M, Nolte I. Co-existence of ununited anconeal process and fragmented medial coronoid process of the ulna in the dog. J Small Anim Prac 2006 47:61-65.
Cook CR, Cook JL. Diagnostic imaging of canine elbow dysplasia: A review. Vet Surg 2009 38:144-153.
Pettitt RA, Tattersall J, Gemmill T, et al. Effect of surgical technique on radiographic fusion of the anconeus in the treatment of ununited anconeal process. J Small Anim Prac 2009 50:545-548.
Temwichitr J, Leegwater PAJ, Hazewinkel HAW. Fragmented coronoid process in the dog: A heritable disease. Vet J 2010 185:123-129.
Trostel CT, McLaughlin RM, Pool RR. Canine lameness caused by developmental orthopedic diseases: fragmented medial coronoid process and ununited anconeal process. Compend Contin Educ Pract Vet 2003 25(2):112-120.
Danielson K, Fitzpatrick N, Muir P, Manley PA. Histomorphometry of fragmented medial coronoid process in dogs: a comparison of affected and normal coronoid processes. Vet Surg 2006 35:501-509.
Moores AP, Benigni L, Lamb CR. Computed tomography versus arthroscopy for detection of canine elbow dysplasia lesions. Vet Surg 2008 37:390-398.
Punke JP, Hulse DA, Kerwin SC, et al. Arthroscopic documentation of elbow cartilage pathology in dogs with clinical lameness without changes on standard radiographic projections. Vet Surg 2009 38:209-212.
Evans RB, Gordon-Evans WJ, Conzemius MG. Comparison of three methods for the management of fragmented medial coronoid process in the dog: A systematic review and meta-analysis. Vet Comp Orthop Traumatol 2008 21:106-109.
Burton NJ, Owen MR, Kirk LS, et al. Conservative versus arthroscopic management for medial coronoid process disease in dogs: A prospective gait evaluation. Vet Surg 2011 40:972-980.
Fitzpatrick N, Yeadon R, Smith T, Schulz K. Techniques of application and initial clinical experience with sliding humeral osteotomy for treatment of medial compartment disease of the canine elbow. Vet Surg 2009 38:261-278.
Conzemius M. Nonconstrained elbow replacement in dogs. Vet Surg 2009 38:279-284.
Trostel CT, McLaughlin RM, Pool RR. Canine Lameness cause by developmental orthopedic diseases: osteochondrosis. Compend Contin Educ Pract Vet 2002 24(11):836-854.
Fitzpatrick N, Yeadon R, Smith TJ. Early clinical experience with osteochondral autograft transfer for treatment of osteochondritis dissecans of the medial humeral condyle in dogs. Vet Surg 2009 38:246-260.
PATELLA LUXATION
L’Eplattenier H, Montavon P. Patellar luxation in dogs and cats: pathogenesis and diagnosis. Compend 2002 24(3)234-239.
Alam MR, Lee JI, Kang HS, et al. Frequency and distribution of patellar luxation in dogs: 134 cases (2000-2005). Vet Comp Orthop Traumatol 2007 20:59-64.
Campbell CA, Horstman CL, Mason DR, Evans RB. Severity of patellar luxation and frequency of concomitant cranial cruciate ligament rupture in dogs: 162 cases (2004-2007). J Am Vet Med Assoc 2010 236:887-891.
L’Eplattenier H, Montavon P. Patellar luxation in dogs and cats: Management and prevention. Compend 2002 24(4)292-299.
Johnson AL, Probst CW, Decamp CE, et al. Comparison of trochlear block recession and trochlear wedge recession for canine patellar luxation using a cadaver model. Vet Surg 2001 30:140-150.
Langenbach A, Marcellin-Little DJ. Management of concurrent patellar luxation and cranial cruciate ligament rupture using modified tibial plateau leveling. J Small Anim Prac 2010 51:97-103.
Yeadon R, Fitzpatrick N, Kowaleski MP. Tibial tuberosity transposition-advancement for treatment of medial patellar luxation and concomitant cranial cruciate ligament disease in the dog. V Comp Orthop Traumatol 2011 24:18-26.